Abstract
Background: Despite the efficacy of venetoclax (VEN) in frontline CLL, optimal combination regimens and duration of treatment remain unclear. We hypothesized that cytoreduction with bendamustine/rituximab (BR) induction followed by venetoclax/rituximab (VR) consolidation for a fixed 1-year duration would be associated with an increased rate of undetectable minimal residual disease (uMRD) compared to historical controls and a reduction in the risk of tumor lysis syndrome (TLS). Here we report data from an ongoing phase 2 multicenter, US, single-arm, open-label study (NCT03609593) designed to assess the safety and efficacy of BR-VR in previously untreated CLL patients (pts).
Methods: Previously untreated CLL/SLL pts ≥ 18 years requiring therapy per iwCLL criteria initially received 3 cycles of bendamustine 50-90 mg/m 2 daily for 2 days and rituximab 375 mg/m 2 every 28 days for 3 cycles. Following BR, VEN was initiated with a standard dose escalation from 20 mg to 400 mg daily over 5 weeks. This was followed by 6 cycles of VR with rituximab given monthly and 5 cycles of VEN alone (12 cycles of VEN in total). Additional eligibility included: ECOG PS ≤ 2, hemoglobin ≥8g/dL, ANC ≥1000/mm 3, and platelets ≥50,000/mm 3. Response was assessed by 2018 iwCLL criteria with uMRD testing by central flow cytometry at a level of <10 -4 in peripheral blood (PB) and bone marrow (BM). The primary endpoint was objective response rate (ORR). Secondary endpoints included uMRD rate, time to uMRD, and adverse events (AEs) assessed by CTCAE v 5.0.
Results: As of data cutoff on 30 May 2021, 26 pts were accrued with additional recruitment ongoing. Baseline demographics were as follows: male/female (16/10), median age 60 yrs (range 44-77). Baseline prognostic studies showed unmutated IGHV in 16 (62%) pts, TP53 aberrant (either del(17p) and/or TP53 mutation) in 1 (4%) pt, del(11q) in 3 (12%) pts, and complex karyotype in 4 (15%) pts. TLS risk among 24 evaluable pts at baseline was high (H) in 3 (12.5%), medium (M) in 15 (62.5%), and low (L) in 6 (25%).
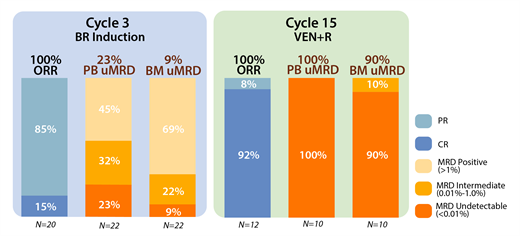
At a median follow-up of 12.9 mo. (range, 1.9-27.5), 23 pts remain on study. Of 12 pts with at least 15 mo. follow-up (completing all therapy), the ORR was 100% (92% CR/CRi, 8% PR [due to small residual nodes]). 3 pts died on study (2 due to COVID-19 and 1 developed newly metastatic squamous cell carcinoma and taken off study after achieving a CR post-VEN ramp-up). Bendamustine was administered at doses of 50 mg/m 2 in 47%, 70 mg/m 2 in 11%, and 90mg/m 2 in 42% of pts. In 20 evaluable pts, response assessments after cytoreduction with BR demonstrated 15% of pts achieved CR/CRi and 85% achieved PR. For evaluable pts at 16 mo., uMRD (<0.01%) in the PB and BM was observed in 100% (10/10) and 90% (9/10) of pts, respectively. MRD was intermediate (0.01% - <1.0%) in 10% (1 patient) in BM (Figure 1 ORR and MRD). Median time to uMRD was 12 mo. (range 3-15) in PB and 14 mo. (range 5.5-15) in BM.
The most common treatment-emergent AEs during BR induction were (any grade/grade ≥3) anemia in 6/2 (21%/7%) pts, nausea in 6/0 (21%/0%), neutropenia in 5/2 (18%/7%), rash in 5/0 (18%/0%), constipation 4/0 (14%/0%), and transaminitis in 3/0 (11%/0%). 2 pts (7%) developed febrile neutropenia during BR. Emergent AEs during VEN treatment included diarrhea in 10/0 (36%/0%) pts, neutropenia in 6/3 (21%/11%), leukopenia in 5/2 (18%/7%), and nausea in 4/0 (14%/0%).
TLS risk was substantially reduced after BR lead-in. Of 3 H-risk pts at baseline, none remained H-risk after BR; of 15 M-risk pts, only 1 remained M-risk, with the remainder at L-risk (94% reduction in H- or M- risk TLS).
Conclusions: BR-VR is a safe and well-tolerated regimen in untreated CLL pts. BR debulking substantially reduces TLS risk, and this sequential strategy achieves high rates of PB and BM uMRD across all prognostic risk groups.
Hill: Celgene (BMS): Consultancy, Honoraria, Research Funding; AstraZenica: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Gentenech: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Support, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; Beigene: Consultancy, Honoraria, Research Funding; Epizyme: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Incyte/Morphysis: Consultancy, Honoraria, Research Funding. Jurcic: AbbVie, BMS/Celgene, Novartis: Consultancy; AbbVie, Arog Pharmaceuticals, Astellas, BMS/Celgene, Forma Therapeutics, Genentech, Gilead Sciences, PTC Therapeutics, Syros Pharmaceuticals: Research Funding. Heaney: CTI: Honoraria, Research Funding; Blueprint: Honoraria, Research Funding; Novartis: Honoraria; Sierra Oncology: Research Funding; Cogent: Research Funding; BMS: Research Funding; Kartos: Research Funding. Lamanna: MingSight Pharmaceuticals, Inc.: Research Funding; Gilead Sciences, Inc.: Consultancy; AbbVie: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Juno Therapeutics, Inc.: Research Funding; Oncternal Therapeutics: Research Funding; Celgene Corporation: Consultancy; Genentech, Inc.: Consultancy, Research Funding; Verastem Oncology: Research Funding; TG Therapeutics, Inc: Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy; BeiGene: Consultancy; Pharmacyclics: Consultancy.
Venetoclax, Bendamustine, and Rituximab are all FDA approved for use in first-line CLL. The combination of these three agents and dosing schedule utilized in this clinical trial is novel and therefore technically reflects an off-label use.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal